Flow Cytometry
Flow Cytometry
Head of service: Dr. Virginia Vila de Sol
E-mail: citometria.hnp@sescam.jccm.es
Flow cytometry is an important tool in biotechnology that is used to simultaneously evaluate physical and biochemical characteristics of single cells at the whole cell level. The multi-parametric analysis begins when cells are stained with fluorescent probes and/or antibodies specific to different cellular parameters. After staining, labeled-cells are acquired and analyzed in a Flow Cytometer, where cells flow one by one through a laser beam. The scattered light - which is cell-specific- and the emitted fluorescence are measured by different detectors that convert light photons into electrical signals, which are subsequently transformed into digital data for computer analysis. In addition to multi-parametric analysis, cell sorting allows physical separation of the cells based on differential parameter expression, which is determined by analytical flow cytometry.
The Flow Cytometry Unit is a facility that provides biotechnology support to the Basic Research Unit of the National Hospital for Paraplegics since 2006. The facility equipment includes a BD FACS Canto II cytometer and a BD FACS Aria IIu cell sorter, both equipped with three laser lines, that permits the detection of up to nine different fluorescence parameters plus two light scattering parameters (FSC and SSC). In addition, the staff of the facility provides scientific support for designing, analyzing and interpreting flow cytometry experiments and generated data to the clinical and basic researchers from our institution, and other public institutions and private companies in the area.
At the Flow Cytometry Unit, we think that flow cytometry education and training is absolutely essential to obtain high quality scientific results, and for this reason we routinely organize different flow cytometry courses in our institution.
Equipment
BD FACS Canto II (BD Biosciences)
Eight fluorescence-detector cytometer. Three laser lines: Blue-488 nm, red-633nm and violet-405 nm. Multi-parametric analysis of heterogeneous cellular complex populations.

BD FACS Aria IIu (BD Biosciences)
Nine fluorescence-detector cell sorter. Three laser lines: Blue-488 nm, red-633nm and violet-405 nm. The cell sorter is equipped with an Automated Cell Deposition Unit (ACDU) that allows to recover sorted-cells in multi-well plates or slides. In addition, the sorter has a temperature control unit that enables the harvest of sorted-cells improving the survival. Temp. range: 4ºC to 42ºC.

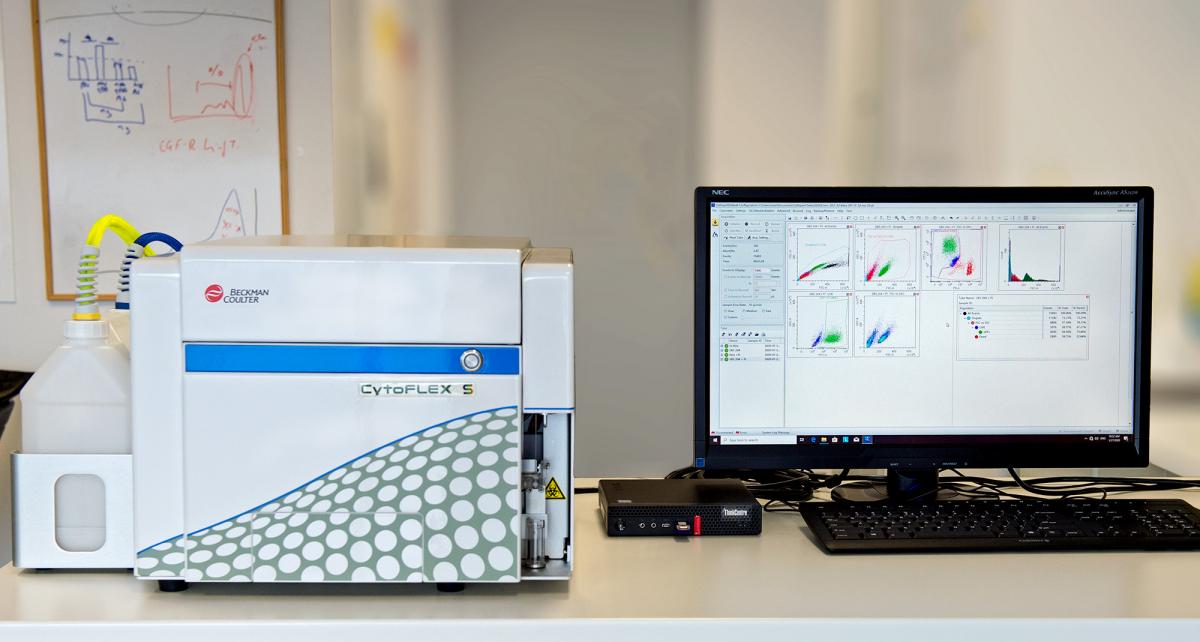
BD FACS CytoFLEX S (Beckman Coulter)
Sixfluorescence-detector cytometer. Three laser lines: Blue-488 nm, yellow/green-561nm and violet-405 nm. Multi-parametric analysis of heterogeneouscellular complex populations. Extracellular Vesicle analysis (80-200 nm). Absolute cell-counter.
MACS System (Miltenyi Biotec)
OctoMACS, for MS columns (up to 108 total cells) and MidiMACS, for LS (up to 109 total cells).
TECHNICAL ASSAYS
- Immunophenotype: Extra and intracellular staining with fluorochrome- coupled antibodies. Multi-parametric analysis up to 9 colours.
- Cell Cycle analysis (Propidium Iodide, Vybrant DyeCycleTM).
- Reporter gene expression analysis (GFP and other fluorescent proteins).
- Proliferation analysis (Tag-It Violet, CFSE, BrdU incorporation assay).
- Apoptosis assays (Anexin V, sub-G1 phase).
- Multiplex assays for soluble molecules (i.e. Cytokines).
- Functional analysis: redox state, phagocytosis, lipid content.
- Fluorescence-Activated Cell Sorting (FACS): rare events at high flow rate, fragile cells sort, etc. Sorting of up to 4 different populations simultaneously. Multi-well plate and slide sorting. Temperature control.
- Magnetic Cell Sorting (MACS): Miltenyi Biotec MACS system.
- Extracellular Vesicles isolation from biofluids.
- Extracellular Vesicles characterization by Flow Cytometry.
Please, click here to know more about our fees
PUBLICATIONS (5 years)
- Reigada D, Nieto-Díaz M, Navarro-Ruiz R, Caballero-López MJ, Del Águila A, Muñoz-Galdeano T, Maza RM."Acute administration of ucf-101 ameliorates the locomotor impairments induced by a traumatic spinal cord injury” Neuroscience. (2015) Aug 6; 300: 404-17. doi: 10.1016/j.neuroscience.2015.05.036.
- Doncel-Pérez E, Mateos-Hernández L, Pareja E, García-Forcada Á, Villar M, Tobes R, Romero Ganuza F, Vila-Del Sol V, Ramos R, Fernández de Mera IG, de la Fuente J. "Expression of Early Growth Response Gene-2 and Regulated Cytokines Correlates with Recovery from Guillain-Barré Syndrome”J Immunol. (2016) Feb 1;196(3):1102-7.doi: 10.4049/jimmunol.1502100
- Moliné-Velázquez V, Vila-Del Sol V, de Castro F, Clemente D. “Myeloid cell distribution and activity in multiple sclerosis” HistolHistopathol. (2016) Apr; 31(4):357-70. doi: 10.14670/HH-11-699.
- Suardíaz M1, Clemente D2, Marin-Bañasco C3, Orpez T3, Hurtado-Guerrero I3, Pavía J4, Pinto-Medel MJ5, De Castro F2, Leyva L5, Fernández O5, Oliver B “Recombinant soluble IFN receptor (sIFNAR2) exhibits intrinsic therapeutic efficacy in a murine model of Multiple Sclerosis” Neuropharmacology. (2016) Nov;110(Pt A):480-492. doi: 10.1016/j.neuropharm.2016.07.026.
- Macrez R, Ortega MC, Bardou I, Mehra A, Fournier A, Van der Pol SM, Haelewyn B, Maubert E, Lesept F, Chevilley A, de Castro F, De Vries HE, Vivien D, Clemente D, Docagne F. “Neuroendothelial NMDA receptors as therapeutic targets in experimental autoimmune encephalomyelitis”Brain. (2016) Sep; 139(Pt 9): 2406-19. doi:10.1093/brain/aww172.
- Reigada D, Navarro-Ruiz RM, Caballero-López MJ, Del Águila Á, Muñoz-Galdeano T, Maza RM, Nieto-Díaz M. "Diadenosinetetraphosphate (Ap4A) inhibits ATP-induced excitotoxicity: a neuroprotective strategy for traumatic spinal cord injury treatment” Purinergic Signal. (2017) Mar;13(1):75-87. doi: 10.1007/s11302-016-9541-4
- Marin-Bañasco C, Benabdellah K, Melero-Jerez C, Oliver B, Pinto-Medel MJ, Hurtado-Guerrero I, de Castro F, Clemente D, Fernández O, Martin F, Leyva L, Suardíaz M. "Gene therapy with mesenchymal stem cells expressing IFN-ß ameliorates neuroinflammation in experimental models of multiple sclerosis” Br J Pharmacol. (2017) Feb; 174(3):238-253. doi: 10.1111/bph.13674.
- de la Cuesta F, Baldan-Martin M, Moreno-Luna R, Alvarez-Llamas G, Gonzalez-Calero L, Mourino-Alvarez L, Sastre-Oliva T, López JA, Vázquez J, Ruiz-Hurtado G, Segura J, Vivanco F, Ruilope LM, Barderas MG. “Kalirin and CHD7: novel endothelial dysfunction indicators in circulating extracellular vesicles from hypertensive patients with albuminuria” Oncotarget. (2017) Feb 28;8(9):15553-15562. doi: 10.18632/oncotarget.14948.
- Mecha M, Feliú A, Machín I, Cordero C, Carrillo-Salinas F, Mestre L, Hernández-Torres G, Ortega-Gutiérrez S, López-Rodríguez ML, de Castro F, Clemente D, Guaza C.” 2-AG limits Theiler's virus induced acute neuroinflammation by modulating microglia and promoting MDSCs” Glia. (2018) Jul; 66(7):1447-1463. doi: 10.1002/glia.23317
- Carolina Melero-Jerez, Margarita Suardíaz, Rafael Lebrón-Galán, Carmen Marín-Bañasco, Begoña Oliver-Martos, Isabel Machín-Díaz, ÓscarFernández, Fernando de Castro, Diego Clemente “The presence and suppressive activity of myeloid-derived suppressor cells are potentiated after interferon-β treatment in a murine model of multiple sclerosis” Neurobiology of Disease (2019) 127:13–31
- David Reigada, Andrés ÁngelCalderón‐García, Manuel Soto‐Catalán, Manuel Nieto‐Díaz, Teresa Muñoz‐Galdeano, Ángela del Águila, Rodrigo M. Maza “MicroRNA-135a-5p reduces P2X7 -dependent rise in intracellular calcium and protects against excitotoxicity” J Neurochem.(2019) Oct; 151(1):116-130.
- González P1, González-Fernández C2, Campos-Martín Y3, Mollejo M3, Carballosa-Gautam M4, Marcillo A4, Norenberg M4, Rodríguez FJ “Frizzled 1 and Wnt1 as new potential therapeutic targets in the traumatically injured spinal cord”Cell Mol Life Sci. (2020) Jan 3. doi: 10.1007/s00018-019-03427-4.
- Hélie, P; Camacho-Toledano, C; Lesec, L; Seillier, C; Miralles, AJ; Ortega, MC; Guérit, S; Lebas, H; Bardou, I; Vila-del Sol, V; Vivien, D; Le Mauff, B; Clemente, D; Docagne, F; Toutirais, O (2021) Tissue plasminogen activator worsens experimental autoimmune encephalomyelitis by complementary actions on lymphoid and myeloid cell responses. J Neuroinflammation 18, 52. https://doi.org/10.1186/s12974-021-02102-5
- Rosa, JM; Farré-Alins, V; Ortega, MC; Navarrete, M; Lopez-Rodriguez, A; Palomino-Antolin, A; Fernandez-Lopez, E; Decouty, C; Narros-Fernández, P; Vila-del Sol, V; Clemente, D; Egea, Javier. (2021) TLR4-pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. British Journal of Pharmacology, DOI: 10.1002/BPH.15488
Staff
Unit Head : Virginia Vila del Sol, PhD
Email: vvila@sescam.jccm.es
Research Technician: Ángela Marquina Rodríguez, MSc
Email: amarquinar@externas.sescam.jccm.es
Lab Technician: María José González López
Email: mjgonzalezl@sescam.jccm.es
Contact
Flow Cytometry Unit
Edif. Investigación Lab. i1-22/23
Hospital Nacional de Parapléjicos
Finca La Peraleda s/n
45071-Toledo
Spain
Email: Citometria.hnp@sescam.jccm.es
Phone. 1: (+34) 925 396 833
Phone. 2: (+34) 925 247 700 Ext. 47168
Fax: (+34) 925 396 821





